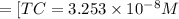Chemistry, 16.03.2020 18:27 21cassitsh
The pH of a water is measured to be 7.5. The system is open to atmosphere and the temperature is 25 oC. Assume that the system is in equilibrium with atmosphere, calculate the concentrations of carbonic acid, bicarbonate, carbonate, and CT (total carbonates). (given pKa1 and pKa2 of H2CO3 are 6.35 and 10.33, respectively).

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2
You know the right answer?
The pH of a water is measured to be 7.5. The system is open to atmosphere and the temperature is 25...
Questions

English, 02.09.2020 23:01

English, 02.09.2020 23:01



Mathematics, 02.09.2020 23:01

Social Studies, 02.09.2020 23:01






Mathematics, 02.09.2020 23:01








![pK_{a1}=-\log[K_{a1}]](/tpl/images/0548/7437/2883e.png)
![6.35=-\log[K_{a1}]](/tpl/images/0548/7437/549c6.png)
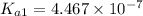
![pK_{a2}=-\log[K_{a2}]](/tpl/images/0548/7437/15d25.png)
![10.33=-\log[K_{a2}]](/tpl/images/0548/7437/898fd.png)
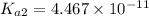
![pH=\log[H^+]](/tpl/images/0548/7437/cd7c9.png)
![7.5=\log[H^+]](/tpl/images/0548/7437/732b6.png)
![[H^+]=3.162\times 10^{-8} M](/tpl/images/0548/7437/5c72c.png)
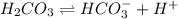
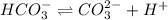
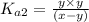
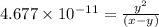 ..[1]
..[1]![x+y=[H^+]](/tpl/images/0548/7437/89af1.png)
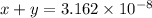 ...[2]
...[2]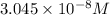
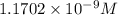
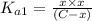
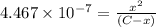
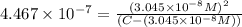
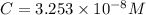
![[H_2CO_3]=(C-x)=3.253\times 10^{-8} M-3.045\times 10^{-8} M](/tpl/images/0548/7437/527d5.png)
![[H_2CO_3]=2.08\times 10^{-9} M](/tpl/images/0548/7437/67449.png)
![[CO_3^{2-}]=y=1.1702\times 10^{-9} M](/tpl/images/0548/7437/122ff.png)
![[HCO_3^{-}]=(x-y)=3.045\times 10^{-8} M-1.1702\times 10^{-9} M=2.928\times 10^{-8} M](/tpl/images/0548/7437/788be.png) Total carbonates:[TC]
Total carbonates:[TC]![[TC]=[H_2CO_3]+[HCO_3^{-}]+[CO_3^{2-}]=C](/tpl/images/0548/7437/a0d4d.png)