

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
An unknown substance has a melting point of 1064°C, is insoluble in water, conducts electricity as a...
Questions

History, 10.11.2020 07:50




Social Studies, 10.11.2020 07:50

Spanish, 10.11.2020 07:50

Mathematics, 10.11.2020 07:50

Chemistry, 10.11.2020 07:50

History, 10.11.2020 07:50

Mathematics, 10.11.2020 07:50


Mathematics, 10.11.2020 07:50


English, 10.11.2020 07:50

English, 10.11.2020 07:50

English, 10.11.2020 07:50

History, 10.11.2020 07:50

Mathematics, 10.11.2020 07:50


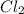 is a gas and it is made up of a non-metal. And, non-metals are poor conductors of heat and electricity therefore,
is a gas and it is made up of a non-metal. And, non-metals are poor conductors of heat and electricity therefore, 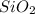 is a non-metallic compound therefore, it is unable to conduct electricity.
is a non-metallic compound therefore, it is unable to conduct electricity. 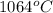 and it is insoluble in water and hard in nature. Also, it is able to conduct electricity in solid state.
and it is insoluble in water and hard in nature. Also, it is able to conduct electricity in solid state.


