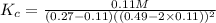Chemistry, 16.03.2020 18:30 Fireburntbudder
This reaction is carried out at a different temperature with initial concentrations of [CO]=0.27M and [H2]=0.49M. At equilibrium, the concentration of CH3OH is 0.11 M. Find the equilibrium constant at this temperature.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
You know the right answer?
This reaction is carried out at a different temperature with initial concentrations of [CO]=0.27M an...
Questions

English, 11.04.2021 21:50


Mathematics, 11.04.2021 21:50


Mathematics, 11.04.2021 21:50


Mathematics, 11.04.2021 21:50



History, 11.04.2021 21:50



Physics, 11.04.2021 21:50

Geography, 11.04.2021 21:50

Mathematics, 11.04.2021 21:50


Geography, 11.04.2021 21:50

Mathematics, 11.04.2021 21:50

English, 11.04.2021 21:50

Mathematics, 11.04.2021 21:50

![[H_2]](/tpl/images/0548/7512/08a38.png) =0.49M.
=0.49M. ![[CH_3OH]=0.11 M](/tpl/images/0548/7512/d5979.png)
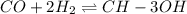
![K_c=\frac{[CH_3OH]}{[CO][H_2]^2}](/tpl/images/0548/7512/4cf94.png)