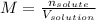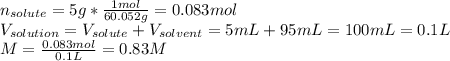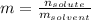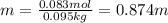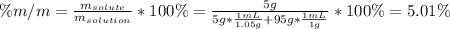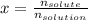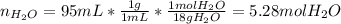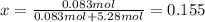Chemistry, 16.03.2020 18:22 cupcake122016
A bottle of commercial vinegar contains 5% acetic acid, ch3cooh, by volume (95% water). the density of acetic acid is 1.05 g/ml and water is 1.00 g/ml. from this data calculate the concentration of acetic acid in vinegar in: molality, molarity, parts by mass, and the mole fraction.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which of the following is a compound? a.carbon b.oxygen c.hydrogen d.water
Answers: 2

Chemistry, 22.06.2019 00:00
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1
You know the right answer?
A bottle of commercial vinegar contains 5% acetic acid, ch3cooh, by volume (95% water). the density...
Questions



Mathematics, 27.08.2020 22:01


History, 27.08.2020 22:01

Physics, 27.08.2020 22:01

Mathematics, 27.08.2020 22:01


Mathematics, 27.08.2020 22:01

Mathematics, 27.08.2020 22:01


English, 27.08.2020 22:01

Arts, 27.08.2020 22:01


Mathematics, 27.08.2020 22:01

Mathematics, 27.08.2020 22:01

History, 27.08.2020 22:01



Social Studies, 27.08.2020 22:01