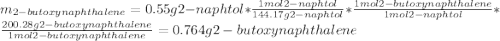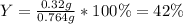Chemistry, 16.03.2020 18:34 daijafoster0
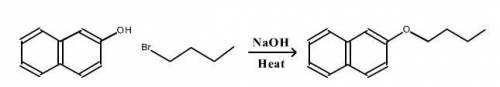
A reaction was performed in which 0.55 g of 2-naphthol was reacted with a slight excess of 1-bromobutane to make 0.32 g of 2-butoxynaphthalene. Calculate the theoretical yield and percent yield for this reaction.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
You know the right answer?
A reaction was performed in which 0.55 g of 2-naphthol was reacted with a slight excess of 1-bromobu...
Questions

Computers and Technology, 29.01.2022 17:00

Computers and Technology, 29.01.2022 17:00

History, 29.01.2022 17:00

Chemistry, 29.01.2022 17:00



Mathematics, 29.01.2022 17:10


Mathematics, 29.01.2022 17:10


History, 29.01.2022 17:10



Mathematics, 29.01.2022 17:10



English, 29.01.2022 17:10

Mathematics, 29.01.2022 17:10


Business, 29.01.2022 17:10