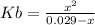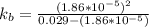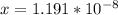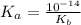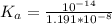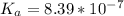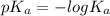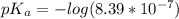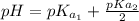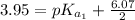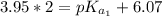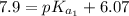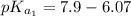Chemistry, 16.03.2020 18:55 winterblanco
The titration of 0.02500 L of a diprotic acid solution with 0.1000 M NaOH requires 34.72 mL of titrant to reach the second equivalence point. The pH is 3.95 at the first equivalence point and 9.27 at the second equivalence point. If the add solution contained 0.2015 g of the acid, what is the molar mass, pK_a1, and pK_a2 of the acid?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1

You know the right answer?
The titration of 0.02500 L of a diprotic acid solution with 0.1000 M NaOH requires 34.72 mL of titra...
Questions

Mathematics, 20.11.2020 20:40


Mathematics, 20.11.2020 20:40


English, 20.11.2020 20:40

Spanish, 20.11.2020 20:40


Engineering, 20.11.2020 20:40

Mathematics, 20.11.2020 20:40

Mathematics, 20.11.2020 20:40

Mathematics, 20.11.2020 20:40

History, 20.11.2020 20:40


History, 20.11.2020 20:40

Mathematics, 20.11.2020 20:40



Mathematics, 20.11.2020 20:40


Health, 20.11.2020 20:40

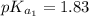
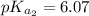
 and
and 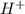 are equal at the equivalence point since they are both taking part in the diprotic acid.
are equal at the equivalence point since they are both taking part in the diprotic acid.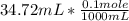
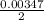 (due to the fact that half of the concentration of NaOH is needed to give the same amount of
(due to the fact that half of the concentration of NaOH is needed to give the same amount of 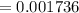
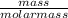
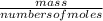
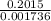
![-log[H^+]](/tpl/images/0548/8178/cbdd4.png)
![[H^+]= 10^{-pH}](/tpl/images/0548/8178/adb7b.png)
![[H^+] = 10^{-9.27](/tpl/images/0548/8178/4fff4.png)
![[H+] = 5.37 * 10^{-10](/tpl/images/0548/8178/27898.png)
![[OH^-]](/tpl/images/0548/8178/b2910.png) can be calculated as follows:
can be calculated as follows:![\frac{10^{-14}}{[H^+]} = \frac {10^{-14}}{5.37* 10^{-10}}](/tpl/images/0548/8178/0ac52.png)
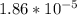
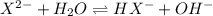
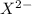 =
= 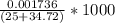
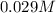

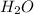
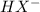
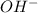
![Kb = \frac{[HX^-][OH^-]}{[X^{2-}]}](/tpl/images/0548/8178/b9431.png)