
Chemistry, 16.03.2020 18:59 nicollexo21
You are instructed to create 900. mL of a 0.29 M phosphate buffer with a pH of 7.8. You have phosphoric acid and the sodium salts NaH2PO4, Na2HPO4, and Na3PO4 available. (Enter all numerical answers to three significant figures.)
H3PO4(s) + H2O(l) <> H3O+(aq) + H2PO4−(aq) Ka1 = 6.9 ✕ 10−3
H2PO4−(aq) + H2O(l) <> H3O+(aq) + HPO42−(aq) Ka2 = 6.2 ✕ 10−8
HPO42−(aq) + H2O(l) <> H3O+(aq) + PO43−(aq) Ka3 = 4.8 ✕ 10−13
Which of the available chemicals will you use for the acid component of your buffer?
A. H3PO4
B. NaH2PO4
C. Na2HPO4
D. Na3PO4
Which of the available chemicals will you use for the base component of your buffer?
A. H3PO4B. NaH2PO4C. Na2HPO4D. Na3PO4

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
You know the right answer?
You are instructed to create 900. mL of a 0.29 M phosphate buffer with a pH of 7.8. You have phospho...
Questions

Health, 24.06.2019 03:50

Biology, 24.06.2019 03:50


Biology, 24.06.2019 03:50


Mathematics, 24.06.2019 03:50

Mathematics, 24.06.2019 03:50


Mathematics, 24.06.2019 03:50


Mathematics, 24.06.2019 03:50


Biology, 24.06.2019 03:50


Biology, 24.06.2019 03:50

English, 24.06.2019 03:50



English, 24.06.2019 03:50

English, 24.06.2019 03:50

![pKa_1=-Log[6.9x10^-^3]=2.16](/tpl/images/0548/8384/6a482.png)
![pKa_2=-Log[6.2x10^-^8]=7.20](/tpl/images/0548/8384/8337b.png)
![pKa_3=-Log[4.8x10^-^13]=12.31](/tpl/images/0548/8384/78632.png)
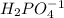 is the acid and
is the acid and 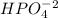 is the base. Therefore the answer for the first question is B and the answer for the second question is C.
is the base. Therefore the answer for the first question is B and the answer for the second question is C.

