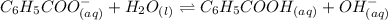Determine if each statement below is True or False regarding Arrhenius and Br∅nsted-Lowry definitions for acids and bases.
1) An Arrhenius acid is a substance that dissolves in water to produce H+ or H3O+.
2) A Br∅nsted-Lowry base is a proton acceptor.
3) CH3COOH is an Arrhenius base.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3


Chemistry, 23.06.2019 05:50
What are the coefficients to balance the following equation? ba+br=babr2
Answers: 1
You know the right answer?
Determine if each statement below is True or False regarding Arrhenius and Br∅nsted-Lowry definition...
Questions


Advanced Placement (AP), 14.01.2021 01:20

Chemistry, 14.01.2021 01:20

Mathematics, 14.01.2021 01:20

Mathematics, 14.01.2021 01:20

English, 14.01.2021 01:20



Mathematics, 14.01.2021 01:20

Arts, 14.01.2021 01:20


Mathematics, 14.01.2021 01:20




Geography, 14.01.2021 01:20



Mathematics, 14.01.2021 01:20

Arts, 14.01.2021 01:20

 .
.