
Chemistry, 16.03.2020 18:56 pierrezonra
An unknown weak acid, HA, it titrated with 1.2 M NaOH. The pH at the halfway point of this titration was found to be 4.081. If the initial pH of the weak acid solution (before titration) has a pH of 2.348, what was the concentration of the weak acid solution

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

You know the right answer?
An unknown weak acid, HA, it titrated with 1.2 M NaOH. The pH at the halfway point of this titration...
Questions

Mathematics, 30.10.2019 15:31


Mathematics, 30.10.2019 15:31

Mathematics, 30.10.2019 15:31




SAT, 30.10.2019 15:31

Mathematics, 30.10.2019 15:31




French, 30.10.2019 15:31

SAT, 30.10.2019 15:31

English, 30.10.2019 15:31

Mathematics, 30.10.2019 15:31


Mathematics, 30.10.2019 15:31

Mathematics, 30.10.2019 15:31

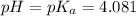 (At halfway point)
(At halfway point)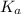 , we use the equation:
, we use the equation: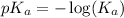
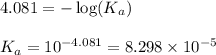
![pH=-\log [H^+]](/tpl/images/0548/8236/37e81.png)
![2.348=-\log [H^+]](/tpl/images/0548/8236/8ea67.png)
![[H^+]=10^{-2.348}=4.487\times 10^{-3}](/tpl/images/0548/8236/4ae47.png)
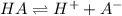
![K_a=\frac{[H^+][A^-]}{[HA]}](/tpl/images/0548/8236/66f51.png)
![[H^+]=[A^-]=4.487\times 10^{-3}](/tpl/images/0548/8236/32c77.png)
![8.298\times 10^{-5}=\frac{(4.487\times 10^{-3})\times (4.487\times 10^{-3})}{[HA]}](/tpl/images/0548/8236/82702.png)
![[HA]=0.243M](/tpl/images/0548/8236/b588d.png)


