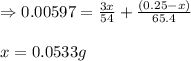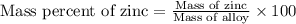Chemistry, 16.03.2020 19:21 juliannabartra
A 0.2500 g sample of an alloy reacts with to form hydrogen gas: 2Al(s) + 6H+(aq) 2Al3+(aq) + 3H2(g) Zn(s) + 2H+(aq) Zn2+(aq) + H2(g) The hydrogen produced has a volume of 0.147 L at 25ºC and 755 mm Hg. What is the percentage of zinc in the alloy

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3
You know the right answer?
A 0.2500 g sample of an alloy reacts with to form hydrogen gas: 2Al(s) + 6H+(aq) 2Al3+(aq) + 3H2(g)...
Questions


Mathematics, 21.05.2021 22:10

Mathematics, 21.05.2021 22:10

English, 21.05.2021 22:10



Mathematics, 21.05.2021 22:10

Geography, 21.05.2021 22:10

Mathematics, 21.05.2021 22:10

Mathematics, 21.05.2021 22:10


Social Studies, 21.05.2021 22:10


Mathematics, 21.05.2021 22:10


Mathematics, 21.05.2021 22:10




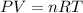
![25^oC=[25+273]K=298K](/tpl/images/0548/8750/df1f6.png)

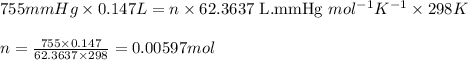
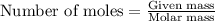 .....(1)
.....(1)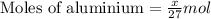
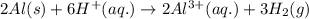
 moles of aluminium will produce =
moles of aluminium will produce = 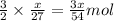 of hydrogen gas
of hydrogen gas
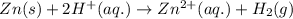
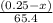 moles of zinc will produce =
moles of zinc will produce = 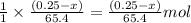 of hydrogen gas
of hydrogen gas