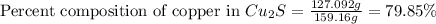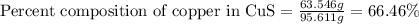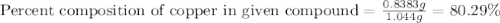Chemistry, 16.03.2020 19:22 justyne2004
In an experiment, a student made a compund of sulfur and copper, and now she needs to find if the compound is copper (I) sulfide or copper (II) sulfide. She cannot physically identify the compound, so she performs a copper analysis, and finds that a 1.0440-gram sample of the compound contains 0.8383 grams of copper. What is the identity of the compund? (HINT: First determine the formulas of the two candidate compounds, and then the percent copper for each one.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 23.06.2019 01:00
Which of the following is a physical change? a.burning a piece of wood b.sawing a piece of wood in half c.rust forming on an iron fence d.a copper roof changing color from orange to green
Answers: 1
You know the right answer?
In an experiment, a student made a compund of sulfur and copper, and now she needs to find if the co...
Questions

Mathematics, 20.03.2021 01:00


Spanish, 20.03.2021 01:00


Mathematics, 20.03.2021 01:00

Mathematics, 20.03.2021 01:00


Mathematics, 20.03.2021 01:00

Mathematics, 20.03.2021 01:00


Mathematics, 20.03.2021 01:00


History, 20.03.2021 01:00



Biology, 20.03.2021 01:00



Mathematics, 20.03.2021 01:00

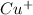 ions and
ions and 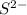 ions
ions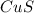 because this is formed by the combination of
because this is formed by the combination of 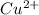 ions and
ions and 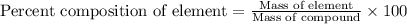 ...........(1)
...........(1)