
Chemistry, 16.03.2020 19:25 mateoperkins
Aluminum is one of the most versatile metals. It is produced by the Hall-Heroult process, in which molten cryolite, Na3AlF6, is used as a solvent for the aluminum ore. Cryolite undergoes very slight decomposition with heat to produce a tiny amount of F2, which escapes into the atmosphere above the solvent. Kc is 2 10-104 at 1300 K for the reaction. Na3AlF6(l) 3 Na(l) Al(l) 3 F2(g) What is the concentration of F2 over a bath of molten cryolite at this temperature

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2


You know the right answer?
Aluminum is one of the most versatile metals. It is produced by the Hall-Heroult process, in which m...
Questions

Chemistry, 13.10.2019 19:50


English, 13.10.2019 19:50


History, 13.10.2019 19:50



Mathematics, 13.10.2019 19:50


Arts, 13.10.2019 19:50


English, 13.10.2019 19:50


Chemistry, 13.10.2019 19:50



Mathematics, 13.10.2019 19:50

Social Studies, 13.10.2019 19:50


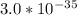
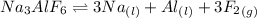
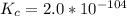
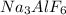
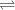
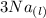

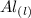
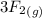
![K_c = [x]^3](/tpl/images/0548/8902/65ef1.png)
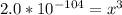
![x =\sqrt[3]{2.0*10^{-104}}](/tpl/images/0548/8902/b2361.png)
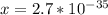
 ≅
≅ 

