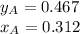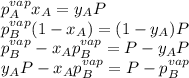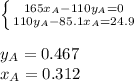Chemistry, 16.03.2020 20:07 minersaysay22
Two volatile liquids A (P A pure= 165 Torr) and B (PB pure= 85.1 Torr) are confined in a piston/cylinder assembly. Initially only the liquid phase is present. As the external pressure is reduced, vapor is first observed at a total pressure of 110 Torr. Calculate the mole fraction of component A in the solution (XA) and the mole fraction of component A in the vapor (YA).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3


Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1
You know the right answer?
Two volatile liquids A (P A pure= 165 Torr) and B (PB pure= 85.1 Torr) are confined in a piston/cyli...
Questions




Computers and Technology, 07.11.2019 05:31




Computers and Technology, 07.11.2019 05:31





Computers and Technology, 07.11.2019 05:31





Computers and Technology, 07.11.2019 05:31


Advanced Placement (AP), 07.11.2019 05:31