
Chemistry, 16.03.2020 21:07 TSZRobloxian8290
The empirical formula of an organic compound is C2H4O. The molecular mass of the compound is 176g/mol.
What is the molecular formula of this compound?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1


Chemistry, 22.06.2019 22:30
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1
You know the right answer?
The empirical formula of an organic compound is C2H4O. The molecular mass of the compound is 176g/mo...
Questions











Social Studies, 27.03.2020 02:14





Health, 27.03.2020 02:14


Computers and Technology, 27.03.2020 02:14


English, 27.03.2020 02:14

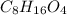 .
.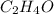 .
.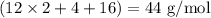
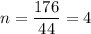
 Empirical formula
Empirical formula 

