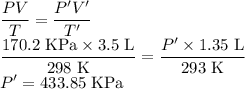Chemistry, 16.03.2020 21:26 michael3592
A sample of ammonia gas at 298 K has a volume of 3.5 L at a pressure of 170.2 kPa, what is the new pressure if the gas is compressed to a volume of 1.35 L at 293 K?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Which term refers to a property that depends only on the amount of a substance? ©@
Answers: 2


Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2
You know the right answer?
A sample of ammonia gas at 298 K has a volume of 3.5 L at a pressure of 170.2 kPa, what is the new p...
Questions











Social Studies, 06.06.2020 04:02









Mathematics, 06.06.2020 04:02