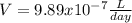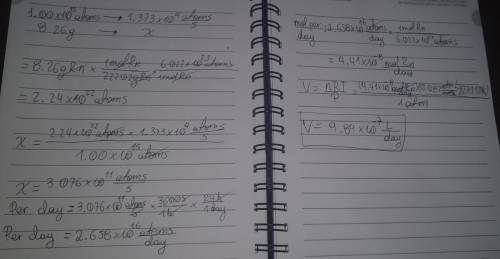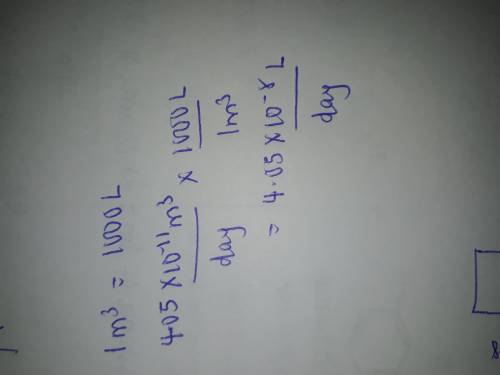Chemistry, 16.03.2020 22:19 dbn4everloved8
Radon (Rn) is the heaviest and the only radioactive member of Group 8A(18), the noble gases. It is a product of the disintegration of heavier radioactive nuclei found in minute concentrations in many common rocks used for building and construction. In recent years, health concerns about the cancers caused from inhaled residential radon have grown. If 1.00 × 1015 atoms of radium (Ra) produce an average of 1.373 × 104 atoms of Rn per second, how many liters of Rn, measured at STP, are produced per day by 8.26 g of Ra?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 23.06.2019 02:30
When the ionic compound nabr dissolves in water, br– ions are pulled into solution by the attraction between what two particles? a. the na+ and br– ions b. the na+ ion and the negative end of a water molecule c. the br– ion and the positive end of a water molecule d. the br– ion and the negative end of a water molecule
Answers: 1

Chemistry, 23.06.2019 06:40
The combustion of methane, ch4, releases 890.4kj/mol. that is, when one mole of methane is burned,890.4 kj are given off to the surroundings. this meansthat the products have 890.4 kj less than the reactants.thus, ah for the reaction = - 890.4 kj. a negative symbolforah indicates an exothermic reaction.ch (g) + 20 (g)> co2 (g) + 2 h0 (1); ah = - 890.4 kga) how much energy is given off when 2.00 mol of ch,are burned? b) how much energy is released when 22.4g of ch. areburned?
Answers: 1
You know the right answer?
Radon (Rn) is the heaviest and the only radioactive member of Group 8A(18), the noble gases. It is a...
Questions

Mathematics, 27.03.2021 18:10

Mathematics, 27.03.2021 18:10

Biology, 27.03.2021 18:10

Biology, 27.03.2021 18:10




Mathematics, 27.03.2021 18:10


Mathematics, 27.03.2021 18:10


Mathematics, 27.03.2021 18:10

Mathematics, 27.03.2021 18:10

Mathematics, 27.03.2021 18:10



History, 27.03.2021 18:10

History, 27.03.2021 18:10

English, 27.03.2021 18:10

Mathematics, 27.03.2021 18:10