Chemistry, 16.03.2020 22:51 needsdarkness6016
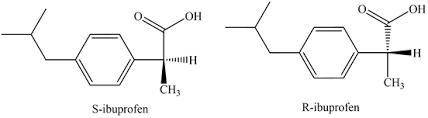
Ibuprofen, a well-known non-steroidal anti-inflammatory drug, has chirality. Only the S enantiomer has anti-inflammatory activity (although the R enantiomer is converted slowly by the body into the S enantiomer). Add wedge-and-dash bonds to complete the perspective structures of the two stereoisomers of ibuprofen.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1


Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1
You know the right answer?
Ibuprofen, a well-known non-steroidal anti-inflammatory drug, has chirality. Only the S enantiomer h...
Questions

Mathematics, 16.09.2019 19:00


Physics, 16.09.2019 19:00



Biology, 16.09.2019 19:00


Medicine, 16.09.2019 19:00

Mathematics, 16.09.2019 19:00

History, 16.09.2019 19:00


Biology, 16.09.2019 19:00


Social Studies, 16.09.2019 19:00

Mathematics, 16.09.2019 19:00

Geography, 16.09.2019 19:00



Social Studies, 16.09.2019 19:00

Health, 16.09.2019 19:00