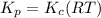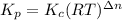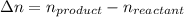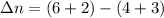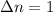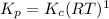Consider the following chemical equilibrium: 4NH3+3O2=2N2+6H2O Now write an equation below that shows how to calculate from for this reaction at an absolute temperature . You can assume is comfortably above room temperature. If you include any common physical constants in your equation be sure you use their standard symbols, found in the ALEKS Calculator.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1
You know the right answer?
Consider the following chemical equilibrium: 4NH3+3O2=2N2+6H2O Now write an equation below that show...
Questions

Mathematics, 13.04.2021 23:40


Mathematics, 13.04.2021 23:40

Arts, 13.04.2021 23:40

Mathematics, 13.04.2021 23:40

Mathematics, 13.04.2021 23:40

History, 13.04.2021 23:40


Biology, 13.04.2021 23:40

Mathematics, 13.04.2021 23:40

Biology, 13.04.2021 23:40

Mathematics, 13.04.2021 23:40

Mathematics, 13.04.2021 23:40

Spanish, 13.04.2021 23:40

History, 13.04.2021 23:40


Biology, 13.04.2021 23:40


English, 13.04.2021 23:40

Mathematics, 13.04.2021 23:40

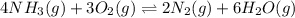
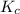 will be,
will be,![K_c=\frac{[N_2]^2[H_2O]^6}{[NH_3]^4[O_2]^3}](/tpl/images/0549/5664/b62d0.png)
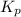 will be,
will be,