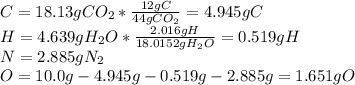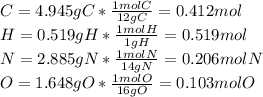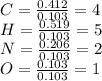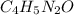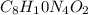Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

You know the right answer?
Caffeine, a molecule found in coffee, tea, and certain soft drinks, contains C, H, O, and N. Combust...
Questions

Mathematics, 23.09.2019 07:30

Biology, 23.09.2019 07:30



History, 23.09.2019 07:30

Biology, 23.09.2019 07:30

Biology, 23.09.2019 07:30


Mathematics, 23.09.2019 07:30

English, 23.09.2019 07:30

Mathematics, 23.09.2019 07:30

Health, 23.09.2019 07:30






Mathematics, 23.09.2019 07:30

English, 23.09.2019 07:30