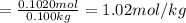Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3

Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3
You know the right answer?
A solution of phosphoric acid was made by dissolving 10.0 g of H3PO4 in 100.0 mL of water. The resul...
Questions

Mathematics, 11.02.2022 18:00

Computers and Technology, 11.02.2022 18:00




History, 11.02.2022 18:00


Mathematics, 11.02.2022 18:00



Mathematics, 11.02.2022 18:10


Mathematics, 11.02.2022 18:10

Mathematics, 11.02.2022 18:10



Mathematics, 11.02.2022 18:10



Mathematics, 11.02.2022 18:10

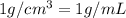
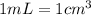
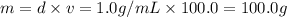
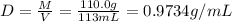
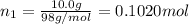
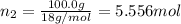
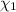
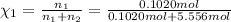
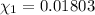
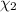
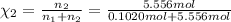
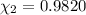
![[Molarity]=\frac{\text{Moles of solute}}{\text{Volume of solution(L)}}](/tpl/images/0549/6266/0dac6.png)
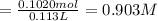
![[Molality]=\frac{\text{Moles of solute}}{\text{Mass of solvent(kg)}}](/tpl/images/0549/6266/71fd2.png)