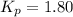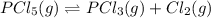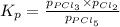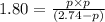Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 22:30
What if it is did darwin used to support his theory of evolution
Answers: 1

Chemistry, 23.06.2019 01:00
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3

Chemistry, 23.06.2019 02:30
Apound is approximately 0.45 kilogram. a persons weighs 87 kilograms. what is the persons’s weight, in pounds, when expressed to the correct number of significant figures
Answers: 1
You know the right answer?
At 250ºC, the equilibrium constant Kp for the reaction PCl5(g) PCl3(g) + Cl2(g) is 1.80. Sufficient...
Questions

Biology, 28.06.2019 11:00


History, 28.06.2019 11:00



Biology, 28.06.2019 11:00




History, 28.06.2019 11:00

English, 28.06.2019 11:00

History, 28.06.2019 11:00


Computers and Technology, 28.06.2019 11:00

Mathematics, 28.06.2019 11:00




Health, 28.06.2019 11:00

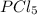 after the system has reached equilibrium.
after the system has reached equilibrium.