
Chemistry, 17.03.2020 00:27 aliyyahlove
In the Haber process, ammonia is synthesized from nitrogen and hydrogen: N2(g) + 3H2(g) → 2NH3(g) ΔG° at 298 K for this reaction is -33.3 kJ/mol. The value of ΔG at 298 K for a reaction mixture that consists of 1.9 atm N2, 1.6 atm H2, and 0.65 atm NH3 is .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1

Chemistry, 23.06.2019 04:00
Why must humans find substitutes for many minerals found on earth? (a) form at an extremely slow rate (b) controlled by other countries (c) too deep in the earth to collect
Answers: 1

You know the right answer?
In the Haber process, ammonia is synthesized from nitrogen and hydrogen: N2(g) + 3H2(g) → 2NH3(g) ΔG...
Questions

Physics, 30.11.2020 20:00

Mathematics, 30.11.2020 20:00

Mathematics, 30.11.2020 20:00

Mathematics, 30.11.2020 20:00







Physics, 30.11.2020 20:00

Biology, 30.11.2020 20:00

Arts, 30.11.2020 20:00

Mathematics, 30.11.2020 20:00

Mathematics, 30.11.2020 20:00



Geography, 30.11.2020 20:00

History, 30.11.2020 20:00

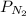 = 1.9 atm
= 1.9 atm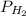 = 1.6 atm
= 1.6 atm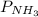 = 0.65 atm
= 0.65 atm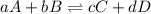
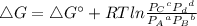
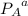 = Partial presser of A
= Partial presser of A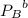 =Partial presser of B
=Partial presser of B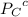 =Partial presser of C
=Partial presser of C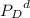 = Partial presser of D
= Partial presser of D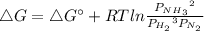
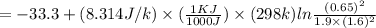 -
-

