
Chemistry, 17.03.2020 00:30 zachzach28280
Be sure to answer all parts. A buffer consists of 0.38 M KHCO3 and 0.71 M K2CO3. Carbonic acid is a diprotic acid with Ka1 = 4.5 × 10−7 and Ka2 = 4.7 × 10−11. (a) Which Ka value is more important to this buffer?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3

Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2
You know the right answer?
Be sure to answer all parts. A buffer consists of 0.38 M KHCO3 and 0.71 M K2CO3. Carbonic acid is a...
Questions

Health, 02.12.2021 04:40

Biology, 02.12.2021 04:40


Mathematics, 02.12.2021 04:40



Mathematics, 02.12.2021 04:40


Geography, 02.12.2021 04:40


History, 02.12.2021 04:40


Physics, 02.12.2021 04:40

Health, 02.12.2021 04:40






Computers and Technology, 02.12.2021 04:40

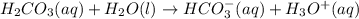
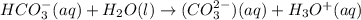
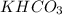 and
and 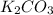 . This means that there will be
. This means that there will be 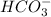 ions and
ions and 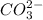 ions and since, both of them are present in the second step.
ions and since, both of them are present in the second step. 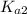 is more significant with respect to this reaction.
is more significant with respect to this reaction. 

