Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1

You know the right answer?
A solution contains 2.0 ⨯ 10−4 M Ag+(aq) and 1.5 ⨯ 10−3 M Pb2+(aq). If NaI is added, will AgI (Ksp =...
Questions




Computers and Technology, 21.01.2020 18:31




Computers and Technology, 21.01.2020 18:31







Arts, 21.01.2020 18:31


Mathematics, 21.01.2020 18:31

History, 21.01.2020 18:31


German, 21.01.2020 18:31

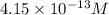 of iodide ion will result in precipitation of silver iodide.
of iodide ion will result in precipitation of silver iodide.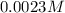 of iodide ion will result in precipitation of lead iodide.
of iodide ion will result in precipitation of lead iodide.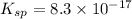
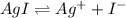
![[Ag^+]=2.0\times 10^{-4} M](/tpl/images/0549/9170/f3b77.png)
![[I^-]](/tpl/images/0549/9170/5a647.png)
![K_{sp}=[Ag^+][I^-]](/tpl/images/0549/9170/9b82b.png)
![[I^-]=\frac{8.3\times 10^{-17}}{2.0\times 10^{-4} M}=4.15\times 10^{-13} M](/tpl/images/0549/9170/c1577.png)
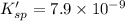
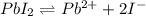
![[Pb^{2+}]=1.5\times 10^{-3} M](/tpl/images/0549/9170/35d28.png)
![K_{sp}'=[Pb^{2+}][I^-]^2](/tpl/images/0549/9170/d5497.png)
![[I^-]^2=\frac{7.9\times 10^{-9}}{1.5\times 10^{-3} M}=4.15\times 10^{-13}](/tpl/images/0549/9170/819e7.png)
![[I^-]=0.0023 M](/tpl/images/0549/9170/2822d.png)