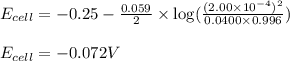Calculate the theoretical potential of the following cell. Indicate whether the reaction will proceed spontaneously in the direction considered (oxidation on the left, reduction on the right) or whether an external voltage source is needed to force this reaction to occur.
Pt, H2(757 torr)|HCl(2.00×10-4 M) parallel to Ni2+(0.0400 M)|Ni
the answer is
-0.072 V; External voltage needed
can anyone explain to me how i get this answer ?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Urea, co(nh2)2, is manufactured on a large scale for use in producing urea-formaldehyde plastics and as a fertilizer. what is the maximum mass of urea that can be manufactured from the co2 produced by combustion of 1.00 x 104 grams of co2?
Answers: 1

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3
You know the right answer?
Calculate the theoretical potential of the following cell. Indicate whether the reaction will procee...
Questions

English, 22.02.2021 20:10


SAT, 22.02.2021 20:10


Mathematics, 22.02.2021 20:10


Computers and Technology, 22.02.2021 20:10


Advanced Placement (AP), 22.02.2021 20:10


Mathematics, 22.02.2021 20:10


Mathematics, 22.02.2021 20:10

Business, 22.02.2021 20:10

Mathematics, 22.02.2021 20:10



History, 22.02.2021 20:10

Mathematics, 22.02.2021 20:10

English, 22.02.2021 20:10

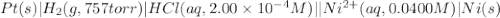
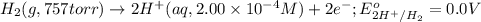
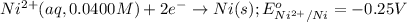

 of the reaction, we use the equation:
of the reaction, we use the equation: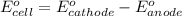
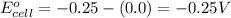
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[H^{+}]^2}{[Ni^{2+}]\times p_{H_2}}](/tpl/images/0549/9866/bc143.png)
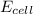 = electrode potential of the cell = ? V
= electrode potential of the cell = ? V![[H^{+}]=2.00\times 10^{-4}M](/tpl/images/0549/9866/69105.png)
![[Ni^{2+}]=0.0400M](/tpl/images/0549/9866/57673.png)
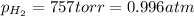 (Conversion factor: 1 atm = 760 torr)
(Conversion factor: 1 atm = 760 torr)