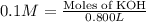Chemistry, 17.03.2020 04:19 theodisb8440
A chemist must prepare 800ml of potassium hydroxide solution with a pH 13 of at 25. He will do this in three steps:
Fill a volumetric flask about halfway with distilled water.
Weigh out a small amount of solid potassium hydroxide and add it to the flask.
Fill the flask to the mark with distilled water.
Calculate the mass of potassium hydroxide that the chemist must weigh out in the second step. Round your answer to significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1
You know the right answer?
A chemist must prepare 800ml of potassium hydroxide solution with a pH 13 of at 25. He will do this...
Questions




Geography, 04.12.2020 09:10




Biology, 04.12.2020 09:10

World Languages, 04.12.2020 09:10



Mathematics, 04.12.2020 09:10


Biology, 04.12.2020 09:10


English, 04.12.2020 09:10


Mathematics, 04.12.2020 09:10


![pOH=-\log[OH^-]](/tpl/images/0550/1622/fe336.png)
![1=-\log[OH^-]](/tpl/images/0550/1622/3f830.png)
![[OH^-]=0.1 M](/tpl/images/0550/1622/e907d.png)
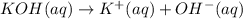
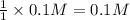 of KOH
of KOH![[Molarity]=\frac{\text{Moles of solute}}{\text{Volume of solution(L)}}](/tpl/images/0550/1622/0dac6.png)