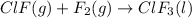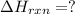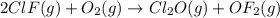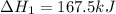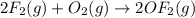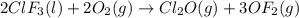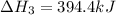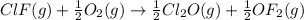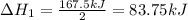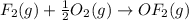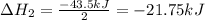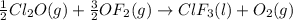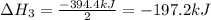Enter your answer in the provided box. Oxidation of gaseous ClF by F2 yields liquid ClF3, an important fluorinating agent. Use the following thermochemical equations to calculate ΔH o rxn for the production of ClF3: (1) 2 ClF(g) + O2(g) → Cl2O(g) + OF2(g) ΔHo = 167.5 kJ (2) 2 F2(g) + O2(g) → 2 OF2(g) ΔHo = −43.5 kJ (3) 2 ClF3(l) + 2 O2(g) → Cl2O(g) + 3 OF2(g) ΔHo = 394.1 kJ

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1

Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3
You know the right answer?
Enter your answer in the provided box. Oxidation of gaseous ClF by F2 yields liquid ClF3, an importa...
Questions





Mathematics, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01



Engineering, 18.10.2020 14:01

Social Studies, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01

English, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01


Geography, 18.10.2020 14:01



Biology, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01

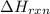 for the reaction is, -135.2 kJ
for the reaction is, -135.2 kJ will be,
will be,