
Chemistry, 17.03.2020 05:26 mixedkiddo
A decorative "ice" sculpture is carved from dry ice (solid CO2) and held at its sublimation point of –78.5°C. Consider the process in which a CO2 sculpture, weighing 389 g, sublimes on a granite tabletop. The temperature of the granite is 12.0°C and the process occurs reversibly. Assume a final temperature for the CO2 vapor of –78.5°C. The enthalpy of sublimation of CO2 is 26.1 kJ/mol. a. Calculate the entropy of sublimation for carbon dioxide (the system) ___ J/Kmol b. Calculate the entropy of the universe for this reversible process. Use three significant figures in the answer __J/K

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3


Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1
You know the right answer?
A decorative "ice" sculpture is carved from dry ice (solid CO2) and held at its sublimation point of...
Questions









History, 14.03.2020 00:53

Computers and Technology, 14.03.2020 00:53


Mathematics, 14.03.2020 00:53

History, 14.03.2020 00:53







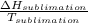
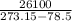
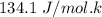
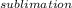 =
= 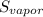 - S
- S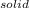 =
= 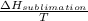
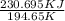
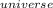 =
= 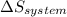 + ΔS
+ ΔS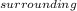
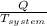
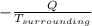
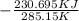 = 1.185 KJ/K - 0.809 KJ/K = 0.376 KJ/K
= 1.185 KJ/K - 0.809 KJ/K = 0.376 KJ/K

