
Chemistry, 17.03.2020 05:24 girdwood797
Duncan knows that it takes 36400 calcal of energy to heat a pint of water from room temperature to boiling. However, Duncan has prepared ramen noodles so many times he does not need to measure the water carefully. If he happens to heat 0.900 pintpint of room-temperature water, how many kilojoules of heat energy will have been absorbed by the water at the moment it begins to boil?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2


Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3
You know the right answer?
Duncan knows that it takes 36400 calcal of energy to heat a pint of water from room temperature to b...
Questions


History, 27.06.2019 11:00

Health, 27.06.2019 11:00

Geography, 27.06.2019 11:00


Mathematics, 27.06.2019 11:00







Mathematics, 27.06.2019 11:00

English, 27.06.2019 11:00

English, 27.06.2019 11:00


Mathematics, 27.06.2019 11:00

Mathematics, 27.06.2019 11:00


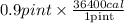
 J
J

