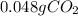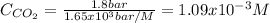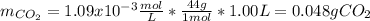Chemistry, 17.03.2020 05:24 adrian128383
How many grams of CO2 are dissolved in a 1.00 L bottle of carbonated water at 298 K if the pressure used in the carbonation process was 1.8 bar? The density of water at this temperature is 998 kg⋅m−3. The Henry's law constant for aqueous solution of CO2 at this temperature is 1.65×103bar.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
You know the right answer?
How many grams of CO2 are dissolved in a 1.00 L bottle of carbonated water at 298 K if the pressure...
Questions

History, 06.04.2021 21:10


Biology, 06.04.2021 21:10








Mathematics, 06.04.2021 21:10


Physics, 06.04.2021 21:10

Mathematics, 06.04.2021 21:10

Mathematics, 06.04.2021 21:10

English, 06.04.2021 21:10




Mathematics, 06.04.2021 21:10