
Chemistry, 17.03.2020 05:41 meramera50
428. mg of an unknown protein are dissolved in enough solvent to make of solution. The osmotic pressure of this solution is measured to be at . Calculate the molar mass of the protein. Round your answer to significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Based on the law of conservation of energy, which statement is false? answer- energy is lost when machines dont work right
Answers: 1

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2
You know the right answer?
428. mg of an unknown protein are dissolved in enough solvent to make of solution. The osmotic press...
Questions

Computers and Technology, 26.05.2021 18:10


Mathematics, 26.05.2021 18:10


Engineering, 26.05.2021 18:10


Chemistry, 26.05.2021 18:10

Mathematics, 26.05.2021 18:10

Physics, 26.05.2021 18:10

English, 26.05.2021 18:10

Mathematics, 26.05.2021 18:10

Mathematics, 26.05.2021 18:10



Mathematics, 26.05.2021 18:10





Mathematics, 26.05.2021 18:10

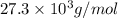
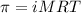
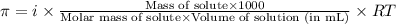
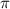 = osmotic pressure of the solution = 0.0766 atm
= osmotic pressure of the solution = 0.0766 atm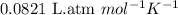
![25^oC=[273+25]=298K](/tpl/images/0550/3624/6a9f9.png)



