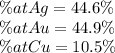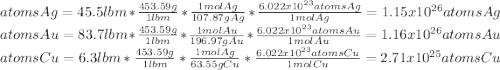Chemistry, 17.03.2020 06:08 elnkun98owvaa6
What is the composition, in atom percent, of an alloy that contains a) 45.5 lbm of silver, b) 83.7 lbm of gold, and c) 6.3 lbm of Cu? The atomic weights for silver, gold, and copper are, respectively, 107.87, 196.97, and 63.55 g/mol.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2
You know the right answer?
What is the composition, in atom percent, of an alloy that contains a) 45.5 lbm of silver, b) 83.7 l...
Questions

Mathematics, 04.07.2019 17:10

Mathematics, 04.07.2019 17:10




Mathematics, 04.07.2019 17:10

Biology, 04.07.2019 17:10

Mathematics, 04.07.2019 17:10

Mathematics, 04.07.2019 17:10


Mathematics, 04.07.2019 17:10


Social Studies, 04.07.2019 17:10




Mathematics, 04.07.2019 17:10



Chemistry, 04.07.2019 17:10