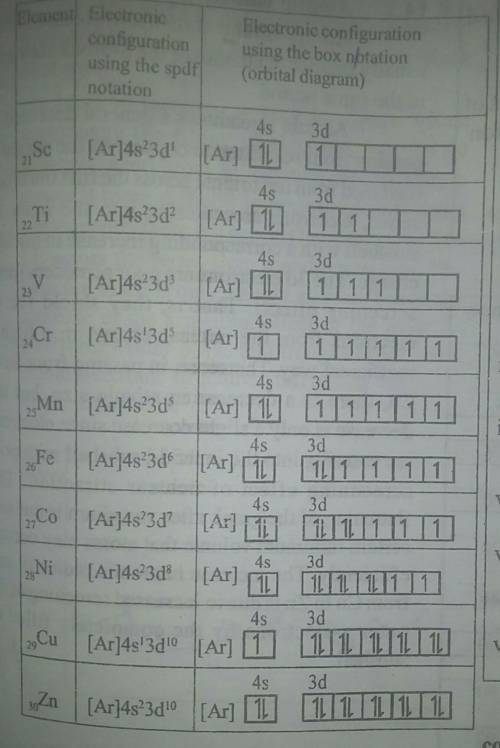
7. (04wP2-5)Elements with atomic number 21 to 30 are d-block elements.
(a) Identify which of t...

Chemistry, 17.03.2020 10:59 chloeethoma24
7. (04wP2-5)Elements with atomic number 21 to 30 are d-block elements.
(a) Identify which of these elements are not considered to be typical transition elements.
12
sh
(b) Complex ions consist of a central metal ion surrounded by ligands. Define the term ligand.
12
(c) Complete the table below to show the oxidation state of the transition element.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1
You know the right answer?
Questions


Mathematics, 03.05.2020 13:06

Mathematics, 03.05.2020 13:06


English, 03.05.2020 13:06

History, 03.05.2020 13:06


English, 03.05.2020 13:06

English, 03.05.2020 13:06

Mathematics, 03.05.2020 13:06


Mathematics, 03.05.2020 13:06

English, 03.05.2020 13:06

Mathematics, 03.05.2020 13:06


English, 03.05.2020 13:06

History, 03.05.2020 13:06


English, 03.05.2020 13:06