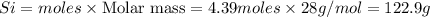The reaction of Cr2O3 with silicon metal at high temperatures will make chromium metal.
...

The reaction of Cr2O3 with silicon metal at high temperatures will make chromium metal.
2Cr2O3(s)+3Si(s) > 4Cr(l)+3SiO2(s)
The reaction is begun with 161.00 g of Si and 139.00 g of Cr2O3.
How many grams of the excess reactant is left after the reaction is complete?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2
You know the right answer?
Questions

History, 05.05.2020 15:49

Mathematics, 05.05.2020 15:49





Business, 05.05.2020 15:49

Mathematics, 05.05.2020 15:49



History, 05.05.2020 15:49






History, 05.05.2020 15:49



Mathematics, 05.05.2020 15:49

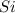 will be left from the given masses of both reactants.
will be left from the given masses of both reactants.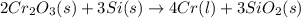
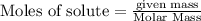
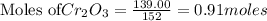
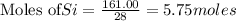
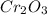 require 3 moles of
require 3 moles of 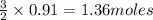 of
of