
Chemistry, 17.03.2020 20:00 davfar334p47luq
Calcium carbonate is an "insoluble salt". But the solubility of CaCO3 can be increased substantially by acidifying the solution. Write all the relevant equilibrium equations and explain why adding acid will increase the solubility of calcium carbonate.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2


Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1
You know the right answer?
Calcium carbonate is an "insoluble salt". But the solubility of CaCO3 can be increased substantially...
Questions

Biology, 03.06.2020 23:01


History, 03.06.2020 23:01

Mathematics, 03.06.2020 23:01

English, 03.06.2020 23:01

Mathematics, 03.06.2020 23:01


Mathematics, 03.06.2020 23:01

Mathematics, 03.06.2020 23:01

Mathematics, 03.06.2020 23:01

History, 03.06.2020 23:01



Mathematics, 03.06.2020 23:01

English, 03.06.2020 23:01

Mathematics, 03.06.2020 23:01

Mathematics, 03.06.2020 23:01


Mathematics, 03.06.2020 23:01

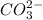 is converted to
is converted to 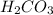 in acidic solution.
in acidic solution.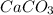 dissociates in solution to produce
dissociates in solution to produce 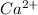 and
and 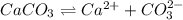
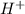 and gets converted to
and gets converted to 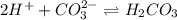 So,
So, 

