
Chemistry, 17.03.2020 21:23 kaiyah2021
Space vehicles use solid lithium hydroxide to remove exhaled carbon dioxide according to the equation: 2LiOH + CO 2 Li 2 CO 3 + H 2 O. Determine the mass of carbon dioxide removed if the space vehicle carries 42.0 mol of LiOH.

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 22:30
For the following, determine the type of reaction and then give products.
Answers: 2

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2
You know the right answer?
Space vehicles use solid lithium hydroxide to remove exhaled carbon dioxide according to the equatio...
Questions

Mathematics, 27.09.2019 14:30


Mathematics, 27.09.2019 14:30

Biology, 27.09.2019 14:30

English, 27.09.2019 14:30

English, 27.09.2019 14:30


Mathematics, 27.09.2019 14:30


Mathematics, 27.09.2019 14:30


History, 27.09.2019 14:30

Social Studies, 27.09.2019 14:30

Advanced Placement (AP), 27.09.2019 14:30


English, 27.09.2019 14:30



Mathematics, 27.09.2019 14:30

Mathematics, 27.09.2019 14:30

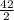 = 21moles of CO₂
= 21moles of CO₂

