
Find the pH of each mixture of acids. Acid Ionization Constants (Ka) for Some Monoprotic Weak Acids at 25 ∘C Acid Formula Ka Benzoic acid HC7H5O2 6.5×10−5 Hydrofluoric acid HF 6.8×10−4 Phenol HC6H5O 1.3×10−10 Formic acid HCHO2 1.8×10−4 Hypochlorous acid HClO 2.9×10−8

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 23.06.2019 04:00
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2

Chemistry, 23.06.2019 04:00
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2
You know the right answer?
Find the pH of each mixture of acids. Acid Ionization Constants (Ka) for Some Monoprotic Weak Acids...
Questions

Mathematics, 18.04.2021 14:00

History, 18.04.2021 14:00


Computers and Technology, 18.04.2021 14:00

Mathematics, 18.04.2021 14:00


Biology, 18.04.2021 14:00

Health, 18.04.2021 14:00

Mathematics, 18.04.2021 14:00



English, 18.04.2021 14:00

Mathematics, 18.04.2021 14:00




Chemistry, 18.04.2021 14:00

Mathematics, 18.04.2021 14:00

Mathematics, 18.04.2021 14:00

World Languages, 18.04.2021 14:00

![[H^+] = \sqrt{K_2C}](/tpl/images/0551/1514/821c9.png)
![[H^+] = \sqrt{6.5*10^{-5}*0.185M}](/tpl/images/0551/1514/28253.png)
![[H^+] =0.0035M](/tpl/images/0551/1514/0669f.png)
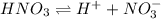
![[H^+]](/tpl/images/0551/1514/07acb.png) = 0.070 M
= 0.070 M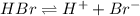
![[H^+] = 0.025 M](/tpl/images/0551/1514/a4539.png)
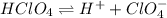
![[H^+] = 0.020 M](/tpl/images/0551/1514/f72cd.png)
![[H^+] = 0.020 M + 0.025 M](/tpl/images/0551/1514/f46a7.png)
![[H^+] = 0.045 M](/tpl/images/0551/1514/4bd75.png)
![[H^+] = \sqrt{K_{a1}C_1+K_{a2}C_2}](/tpl/images/0551/1514/9f22b.png)
![[H^+] = \sqrt{6.8*10^{-4}*0.095 +1,3*10^{-10}*0.230](/tpl/images/0551/1514/a064b.png)
![[H^+] = 0.008037 M](/tpl/images/0551/1514/33641.png)


