Chemistry, 17.03.2020 21:42 dzepeda061
2 AgNO3(aq) + CaCl2(aq) > 2 AgCl(s) + Ca(NO3)2(aq)
How many grams of AgCi are formed when 0.200 L of 0.200 M AGNO3 reacts with an excess of CaCl2?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1


Chemistry, 23.06.2019 06:00
In an exothermic reaction at equilibrium, what is the effect of lowering the temperature? a. the reaction makes more products. b. the reaction makes more reactants. c. the reaction is unchanged.
Answers: 1

Chemistry, 23.06.2019 14:00
Tinererining 01: 57: 44 which statement correcte describes the actual veld and the theoretical yield of a reaction? textual vec is calculated to the reactant amounts but the theoretical yeld must be measured for each instance of a the actual vec is calculated from the amount of the limiting reactant and the theoretical yield is calculated from the 发公主 the actual weld depends on the reaction centers, but the theoretical yield and only with reactant amounts the actual vele represents the maximum weld possible and the theoretical yield assumes perfect reaction conditions save and ext e அட
Answers: 2
You know the right answer?
2 AgNO3(aq) + CaCl2(aq) > 2 AgCl(s) + Ca(NO3)2(aq)
How many grams of AgCi are formed...
How many grams of AgCi are formed...
Questions


Mathematics, 24.11.2019 05:31


Mathematics, 24.11.2019 05:31






Biology, 24.11.2019 05:31

History, 24.11.2019 05:31


Physics, 24.11.2019 05:31

Mathematics, 24.11.2019 05:31

Mathematics, 24.11.2019 05:31



Mathematics, 24.11.2019 05:31

Mathematics, 24.11.2019 05:31

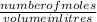
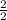 =
=