
What mass of silver chloride can be produced from 1.30 L of a 0.245 M solution of silver nitrate? The reaction described in Part A required 3.36 L of calcium chloride. What is the concentration of this calcium chloride solution?
Please explain, I don't know where to begin.
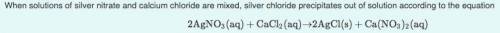

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
One mole of zinc has a mass of 65.4 grams. approximately how many atoms of zinc are present in one mole of zinc? 32 × 1023 atoms 6 × 1023 atoms 66 atoms 65 atoms
Answers: 1

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2
You know the right answer?
What mass of silver chloride can be produced from 1.30 L of a 0.245 M solution of silver nitrate? Th...
Questions


Mathematics, 28.07.2021 14:00


Mathematics, 28.07.2021 14:00

Computers and Technology, 28.07.2021 14:00


Business, 28.07.2021 14:00

Biology, 28.07.2021 14:00


Health, 28.07.2021 14:00


English, 28.07.2021 14:00

Mathematics, 28.07.2021 14:00

English, 28.07.2021 14:00

English, 28.07.2021 14:00


Mathematics, 28.07.2021 14:00

Mathematics, 28.07.2021 14:00

Mathematics, 28.07.2021 14:00

Mathematics, 28.07.2021 14:00



