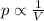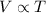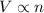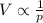Chemistry, 17.03.2020 22:32 angmendezdiaz
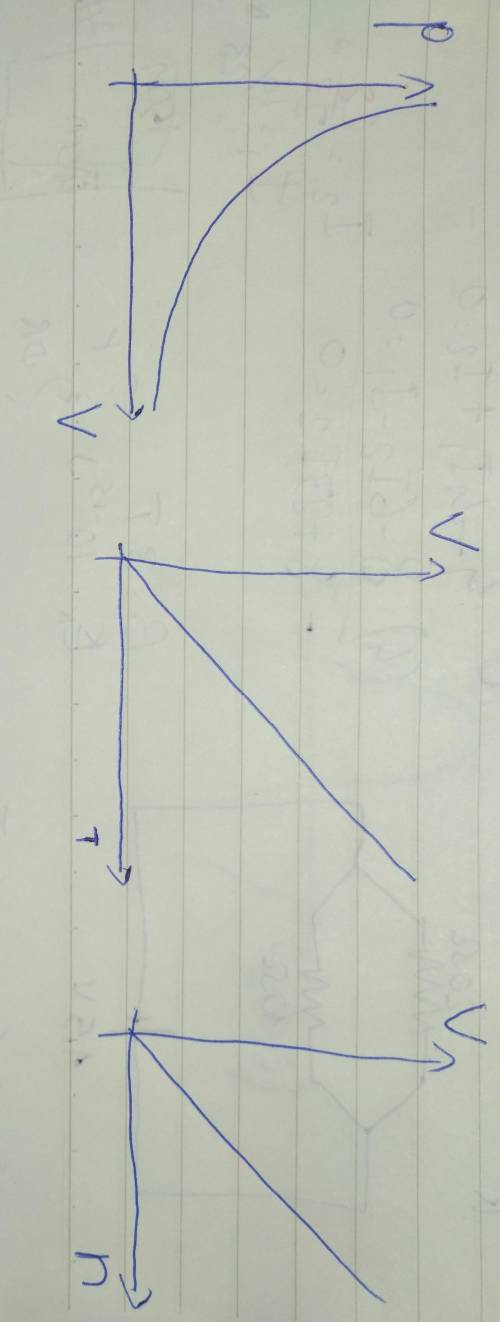
Compare Boyle's law, Charles's law, and Avogadro's law. a. What remains constant in each law? b. What are the variables in each law? c. What do the graphs for these laws look like? d. Write each law with V isolated. Using these equations and the graphs from part c, which law(s) show a directly proportional relationship? How can you tell?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3


Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
Compare Boyle's law, Charles's law, and Avogadro's law. a. What remains constant in each law? b. Wha...
Questions

Chemistry, 11.07.2019 17:30

Mathematics, 11.07.2019 17:30


Biology, 11.07.2019 17:30

Mathematics, 11.07.2019 17:30

Mathematics, 11.07.2019 17:30


Mathematics, 11.07.2019 17:30





Mathematics, 11.07.2019 17:30

Mathematics, 11.07.2019 17:30

Computers and Technology, 11.07.2019 17:30



Mathematics, 11.07.2019 17:30

Physics, 11.07.2019 17:30

Mathematics, 11.07.2019 17:30