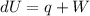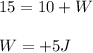Chemistry, 18.03.2020 00:44 littlesami105
If we added heat (Q) to the, and we knew this resulted in an increase of internal energy (U) of + 15, what would the change in work (W) have to be in order for the equation to balance.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2
You know the right answer?
If we added heat (Q) to the, and we knew this resulted in an increase of internal energy (U) of + 15...
Questions

Mathematics, 18.08.2019 10:30


History, 18.08.2019 10:30

Mathematics, 18.08.2019 10:30


History, 18.08.2019 10:30

Mathematics, 18.08.2019 10:30


Mathematics, 18.08.2019 10:50


Social Studies, 18.08.2019 10:50

Biology, 18.08.2019 10:50

Mathematics, 18.08.2019 10:50


Chemistry, 18.08.2019 10:50




Physics, 18.08.2019 10:50

Mathematics, 18.08.2019 10:50