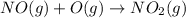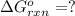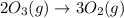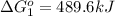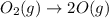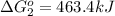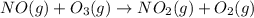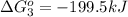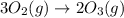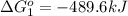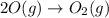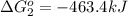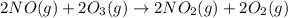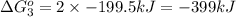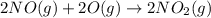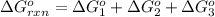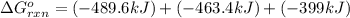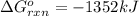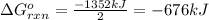Chemistry, 18.03.2020 17:20 ishaanbaruah4474
Use Hess's law to calculate ΔG°rxn using the following information. NO(g) + O(g) → NO2(g) ΔG°rxn = ? 2 O3(g) → 3 O2(g) ΔG°rxn = +489.6 kJ O2(g) → 2 O(g) ΔG°rxn = +463.4 kJ NO(g) + O3(g) → NO2(g) + O2(g) ΔG°rxn = -199.5 kJ

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2
You know the right answer?
Use Hess's law to calculate ΔG°rxn using the following information. NO(g) + O(g) → NO2(g) ΔG°rxn = ?...
Questions

Mathematics, 06.05.2020 00:41

Chemistry, 06.05.2020 00:41

Mathematics, 06.05.2020 00:41

Mathematics, 06.05.2020 00:41







English, 06.05.2020 00:41






Mathematics, 06.05.2020 00:41

Mathematics, 06.05.2020 00:41

Mathematics, 06.05.2020 00:41

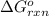 is, -676 kJ
is, -676 kJ