
Chemistry, 18.03.2020 18:32 laceysmith2i023
Al(OH)3 + OH- → Al(OH)4- Identify whether this reaction can be classified as a Bronsted-Lowry and/or Lewis acid/base reaction. Group of answer choices Only Bronsted-Lowry Only Lewis Both Bronsted-Lowry and Lewis Neither

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1
You know the right answer?
Al(OH)3 + OH- → Al(OH)4- Identify whether this reaction can be classified as a Bronsted-Lowry and/or...
Questions

Biology, 26.03.2021 17:10




Mathematics, 26.03.2021 17:10




Mathematics, 26.03.2021 17:10


Mathematics, 26.03.2021 17:10

Business, 26.03.2021 17:10

Computers and Technology, 26.03.2021 17:10

Mathematics, 26.03.2021 17:10

Mathematics, 26.03.2021 17:10


History, 26.03.2021 17:10

Mathematics, 26.03.2021 17:10

Biology, 26.03.2021 17:10

Mathematics, 26.03.2021 17:10

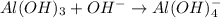
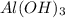 is a Lewis acid because it is an electron deficient species that means, it accepts electron pairs.
is a Lewis acid because it is an electron deficient species that means, it accepts electron pairs.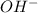 is a Lewis base because it is an electron rich species that means, it donates electron pairs.
is a Lewis base because it is an electron rich species that means, it donates electron pairs.

