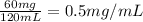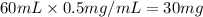Chemistry, 18.03.2020 18:54 esanchez2002fcb
G During a coarse titration, you placed 60 mg of unknown acid in 120 mL water, and dispensed 20 mL of NaOH to this acid solution (analyte) to reach the endpoint. What should be the mass and volume of the analyte so it takes 10 mL of NaOH to reach the endpoint

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 23.06.2019 06:40
4786 joules of heat are transferred to a 89.0 gramsample of an unknown material, with an initialtemperature of 23.0°c. what is the specific heat of thematerialif the final temperature is 89.5 °c?
Answers: 1
You know the right answer?
G During a coarse titration, you placed 60 mg of unknown acid in 120 mL water, and dispensed 20 mL o...
Questions



Mathematics, 03.11.2020 14:00


Mathematics, 03.11.2020 14:00

Mathematics, 03.11.2020 14:00

History, 03.11.2020 14:00


Mathematics, 03.11.2020 14:00

Mathematics, 03.11.2020 14:00


Mathematics, 03.11.2020 14:00

Mathematics, 03.11.2020 14:00

Computers and Technology, 03.11.2020 14:00


Social Studies, 03.11.2020 14:00

History, 03.11.2020 14:00


Geography, 03.11.2020 14:00

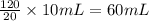 unknown acid solution.
unknown acid solution.