
Chemistry, 18.03.2020 20:44 morganhines181
Predict the sign of ΔS° for 2NO2(g) LaTeX: \longrightarrow⟶ N2O4(g) CaCO3(s) + 2HCl(aq) LaTeX: \longrightarrow⟶ CaCl2(aq) + H2O(l) +CO2(g) Ag+(aq) + Cl-(aq) LaTeX: \longrightarrow⟶ AgCl(s)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2


Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
You know the right answer?
Predict the sign of ΔS° for 2NO2(g) LaTeX: \longrightarrow⟶ N2O4(g) CaCO3(s) + 2HCl(aq) LaTeX: \long...
Questions

Mathematics, 01.05.2021 04:10

Mathematics, 01.05.2021 04:10

Mathematics, 01.05.2021 04:10

Spanish, 01.05.2021 04:10

Biology, 01.05.2021 04:10


Mathematics, 01.05.2021 04:10






Computers and Technology, 01.05.2021 04:10


Mathematics, 01.05.2021 04:10



English, 01.05.2021 04:10

Mathematics, 01.05.2021 04:10

English, 01.05.2021 04:10

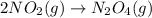 :
: 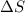 is negative
is negative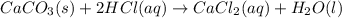 :
: 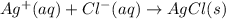 :
: 

