
Chemistry, 18.03.2020 21:34 odriskel49
An aqueous solution of calcium hydroxide is standardized by titration with a 0.164 M solution of hydroiodic acid. If 17.2 mL of base are required to neutralize 14.7 mL of the acid, what is the molarity of the calcium hydroxide solution?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2


Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3
You know the right answer?
An aqueous solution of calcium hydroxide is standardized by titration with a 0.164 M solution of hyd...
Questions

Geography, 04.05.2021 22:20

French, 04.05.2021 22:20

Chemistry, 04.05.2021 22:20


Mathematics, 04.05.2021 22:20


Mathematics, 04.05.2021 22:20


History, 04.05.2021 22:20

Mathematics, 04.05.2021 22:20

Social Studies, 04.05.2021 22:20

Mathematics, 04.05.2021 22:20

Mathematics, 04.05.2021 22:20

Mathematics, 04.05.2021 22:20

Mathematics, 04.05.2021 22:20

Mathematics, 04.05.2021 22:20

Mathematics, 04.05.2021 22:20


Mathematics, 04.05.2021 22:20




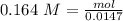
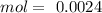
 : 2 mol of
: 2 mol of 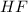 ). With this molar ratio, we can find the moles of
). With this molar ratio, we can find the moles of 




