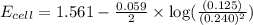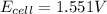Chemistry, 18.03.2020 21:41 cheerthi16
The standard reduction potentials for the Ag+|Ag(s) and Zn2+| Zn(s) half-cell reactions are +0.799 V and -0.762 V, respectively. Calculate the potential for the following electrochemical cell: Zn(s)|Zn2+(0.125 M)||Ag+(0.240 M)|Ag(s)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1
You know the right answer?
The standard reduction potentials for the Ag+|Ag(s) and Zn2+| Zn(s) half-cell reactions are +0.799 V...
Questions



Mathematics, 03.08.2019 01:20



Mathematics, 03.08.2019 01:20

Mathematics, 03.08.2019 01:20













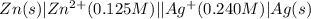
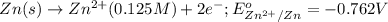
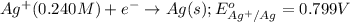 ( × 2)
( × 2)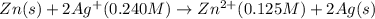
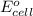 of the reaction, we use the equation:
of the reaction, we use the equation: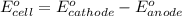
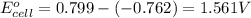
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[Zn^{2+}]}{[Ag^{+}]^2}](/tpl/images/0552/5842/bffc2.png)
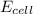 = electrode potential of the cell = ? V
= electrode potential of the cell = ? V![[Zn^{2+}]=0.125M](/tpl/images/0552/5842/c12f1.png)
![[Ag^{+}]=0.240M](/tpl/images/0552/5842/c82de.png)