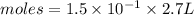Chemistry, 18.03.2020 23:01 AlwaysMarcus5577
For the reaction 2 H2O(g) equilibrium reaction arrow 2 H2(g) + O2(g), K = 2.4 ✕ 10−3 at a given temperature. At equilibrium in a 2.7 L container, it is found that [H2O(g)] = 2.0 ✕ 10−1 M and [H2(g)] = 2.5 ✕ 10−2 M. Calculate the moles of O2(g) present under these conditions.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2


Chemistry, 23.06.2019 02:30
What type of energy conversion occurs when you place your feet near the fire place and they become warm
Answers: 1

Chemistry, 23.06.2019 03:30
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1
You know the right answer?
For the reaction 2 H2O(g) equilibrium reaction arrow 2 H2(g) + O2(g), K = 2.4 ✕ 10−3 at a given temp...
Questions



SAT, 16.01.2021 22:00

Mathematics, 16.01.2021 22:00



Mathematics, 16.01.2021 22:00

Mathematics, 16.01.2021 22:00

Biology, 16.01.2021 22:00



Mathematics, 16.01.2021 22:00



Arts, 16.01.2021 22:00


English, 16.01.2021 22:00



Mathematics, 16.01.2021 22:00

 moles of
moles of 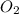 will be produced.
will be produced.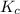 will be as follows,
will be as follows,![K_{c} = \frac{[H_{2} ]^2[O_{2} ]}{[H_{2}O ]^2 }](/tpl/images/0552/7877/22d2b.png)
![2.4\times10^{-2} = \frac{[2.5\times10^{-2} ]^2[O_{2} ]}{[2\times10^{-1} ]^2 }](/tpl/images/0552/7877/022e7.png)
![[O_{2} ]=\frac{[2.4\times10^{-3}][2\times10^{-1} ]^2 }{[2.5\times10^{-2} ]^2}](/tpl/images/0552/7877/7587e.png)
![[O_{2}]= 1.5\times10^{-1}M](/tpl/images/0552/7877/aba91.png)
![[O_{2} ]= \frac{moles}{LItre}](/tpl/images/0552/7877/7e5ff.png)
![[1.5\times10^{-1} ]= \frac{moles}{2.7}](/tpl/images/0552/7877/f2d4f.png)