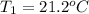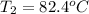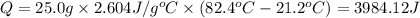Chemistry, 19.03.2020 00:24 samueldfhung
How many joules of heat are required to heat 25.0 g of isopropyl alcohol from the prevailing room temperature, 21.2 oC, to its boiling point, 82.4 oC? The specific heat of isopropyl alcohol is 2.604 J/g °C.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1

Chemistry, 23.06.2019 05:20
Explain how global warming could have affected yellowstone frog and salamander habitat's, resulting in changes in the populations of these species
Answers: 2
You know the right answer?
How many joules of heat are required to heat 25.0 g of isopropyl alcohol from the prevailing room te...
Questions

English, 20.09.2020 22:01

English, 20.09.2020 22:01

Mathematics, 20.09.2020 22:01

Mathematics, 20.09.2020 22:01

English, 20.09.2020 22:01


Mathematics, 20.09.2020 22:01

History, 20.09.2020 22:01

English, 20.09.2020 22:01

Mathematics, 20.09.2020 22:01

Mathematics, 20.09.2020 22:01


English, 20.09.2020 22:01


Mathematics, 20.09.2020 22:01

Mathematics, 20.09.2020 22:01



Chemistry, 20.09.2020 22:01

Mathematics, 20.09.2020 22:01

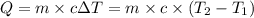
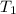 = Initial temperature of the substance
= Initial temperature of the substance 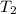 = Final temperature of the substance
= Final temperature of the substance