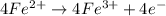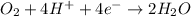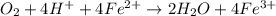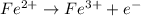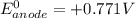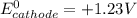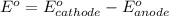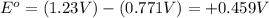Chemistry, 19.03.2020 00:52 gabischmid4340
A chemist designs a galvanic cell that uses these two half-reactions:
Half-reaction Standard reduction potential
O₂(g) + 4H⁺(aq) + 4e⁻ → 2H₂O(l) E⁰ red = +1.23 V
Fe³⁺(aq) + e⁻ → Fe²⁺(aq) E⁰ red = +0.771 V
(a) Write a balanced equation for the half-reaction that happens at the cathode.
(b) Write a balanced equation for the half-reaction that happens at the anode.
(c) Write a balanced equation for the overall reaction that powers the cell. Be sure the reaction is spontaneous as written.
(d) Do you have enough information to calculate the cell voltage under standard conditions?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 23.06.2019 04:31
2ki + pb(no3)2 → 2kno3 + pbi2 determine how many moles of kno3 are created if 0.03 moles of ki are completely consumed.
Answers: 1

Chemistry, 23.06.2019 06:20
Type the correct answer in each box.balance the chemical equation.__ n203 ➡️ __ n2 +__ o2
Answers: 1

Chemistry, 23.06.2019 09:30
Large crystals are formed when igneous rocks cool very slowly igneous rocks cool very quickly sedimentary rock is eroded metamorphic rocks change into igneous rock
Answers: 1
You know the right answer?
A chemist designs a galvanic cell that uses these two half-reactions:
Half-reaction Stan...
Half-reaction Stan...
Questions








History, 16.07.2019 02:30


Mathematics, 16.07.2019 02:30

Mathematics, 16.07.2019 02:30

Mathematics, 16.07.2019 02:30





English, 16.07.2019 02:30



Mathematics, 16.07.2019 02:30